Tuesday, August 12
I was (finally) able to organize some things for my project today. First, I finished my 24-hour sampling time point of the feeding trial. Then, I organized all of my samples into appropriate boxes. I can’t bring any coral samples with me when I leave this time because our CITES permit is pending, so I counted all the Symbiodiniaceae cells of the coral samples under the microscope. Then, I used our 3D scanner to make models of all the coral fragments and calculate their surface area. Combined with the cell counts, this will let me calculate the exact number of live/dead Symbiodiniaceae cells I fed the roris during the feeding trial, which I’ll compare with the number of cells in the rori poop.

I scanned coral fragments with our fancy 3D scanner.
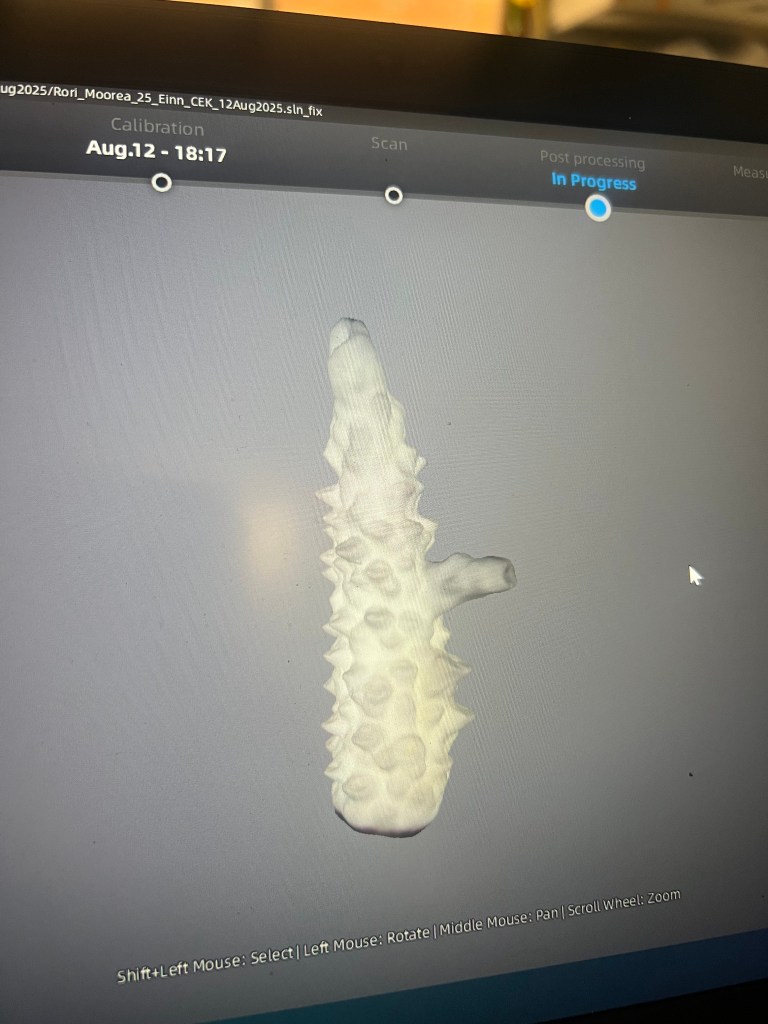

The scanner creates a 3D model (left) of coral frags (right).
Monday, August 11
Today was my final coral feeding trial of the trip! We had a very successful day of sampling and sample processing, and I’m feeling excited to analyze all our data back at Cal 🙂

Here’s a snapshot of all the samples we’ve collected during these feeding trials – ooooh, aaaaah. Fear not, these are only some of MANY we’ve collected this trip (remember the 80 samples?)
Sunday, August 10
Woohoo, today was a special day because it was my birthday!! I’m so lucky to have such amazing coworkers/labmates/friends who celebrated with me with a morning run; quick run to the poop site to collect more rori; call with my family; and an evening of pizza, cake, and Wicked 🙂

I collected four more sea cucumbers for my next feeding trial tomorrow.
Saturday, August 9
After finishing up my 24-hour processing day, I took the rest of the day to rest up. It ended with happy hour and a beautiful sunset with the lab 🙂

The sunset was beautiful today.
Friday, August 8
Wow! Today was wild!! We had a big boat day today, sampling at three different sites on the West and North shores of Mo’orea. We ended up collecting ~80 (yes, 80!!!) samples. We got back to the lab around 3 pm to start sample processing. Ariana Grande’s entire discography and 4+ musical soundtracks later…we finally finished processing all of these samples around 8 am the next morning. 24 full hours of work, but hopefully it’s worth it after we get our sequencing data back!
The most exciting part was seeing a rori feeding on sand directly by a coral and pooping on one!! Talk about opportunities for trophic transmission 😛

I saw a rori pooping directly on a coral today!
Thursday, August 7
We did a few more in situ sample collections today. Up to this point, I’ve been collecting only fresh poos (that I see leave the rori). To ramp up my sample size before I leave next week and to add another dimension to compare between samples, I’m going to experiment with collecting fresh-looking poos (i.e., intact poos near the rori’s butt) tomorrow.

We collected more rori poop samples today.
Wednesday, August 6
I started my day by collecting the final, 24 hour time point for my feeding trial yesterday. Then, I got an afternoon off, yay!!
Tuesday, August 5
Today I started a new feeding trial, which Jason Spingarn-Koff was kind enough to film! Thanks, Jason! I decided to slightly modify the protocol, so I took a sample from each sea cucumber every hour for 8 hours, then again after 12 and 24 hours of coral addition. Instead of collecting every individual poop, I grouped them by the hour/time point. I also changed my filtering method to only cell strain since we’ve been having issues with some new meshes we bought for this trip. I’ll finish the filtering process back at Cal to process these samples under the microscope.
Monday, August 4
I went to a new site today to collect some in situ samples (on the reef). I collected pairs of rori poop and sediment near the rori’s mouth. Here are some pictures I Took of the site and sea cucumbers hanging out on/near coral.



Sunday, August 3
I returned the sea cucumbers from the feeding trial to the reef today to prepare for another feeding trial. I didn’t take many photos today, but we got some amazing professional photos from the boat. Enjoy this photo of me and my advisor!

Jason Spingarn-Koff joined us on the boat to document some of our rori processing!
I also analyzed samples from yesterday’s feeding trial under the microscope and decided to make some changes to my sampling protocol before the next one.
Saturday, August 2
I started out my day with a cup of coffee to prepare for a whole day of sampling!

I started my day with some much-needed caffeine.
Then, I got started with the feeding trial. First, I cut pieces of coral and airbrushed tissue off of them. Then, I fed this to the sea cucumbers with a syringe, sort of like feeding a baby with a bottle.

We feed the coral tissue slurry to the sea cucumbers with syringes.
Then, we monitored the sea cucumbers for five hours and collected every single poop. I’m interested in whether I see large amounts of live (or dead) Symbiodiniaceae cells in any of these samples.


We watched the rori for five hours and collected every…single…poop!
We powered our sampling day with lots of candy and chocolate milk 🙂
The sample processing starts by weighing the samples. Then, I filter them through a cell strainer + mesh apparatus before preserving them.
The day was super long, but we did it! I’m going to analyze our data soon and potentially tweak the sampling method.
Friday, August 1
I set up my tanks for a coral feeding trial tomorrow. This means that I got to collect four rori from the reef! While doing this, I saw an octopus!! :-0

I saw an octopus today!
Tomorrow I’m planning a semi-wild day! I’m going to feed sea cucumbers coral tissue slurry and collect every single poop for five hours. Stay tuned!
Thursday, July 31
I started my day with a morning run!

I had a beautiful view on my run.
Then, I ran another PCR to see whether I could find bacterial DNA in my samples. Based on those results, I adjusted my sampling protocol a bit for future sample collections.
Wednesday, July 30
Today I tested whether Symbiodiniaceae DNA was present in my samples by using polymerase chain reaction (PCR), which makes a bunch of copies of particular segments of DNA. Then, I ran my PCR product on an agarose gel with gel electrophoresis. If there are bands present at ~300 bp, then I know my PCR worked and that I found Symbiodiniaceae DNA in my samples!
However, my gel was looking a little bit sad because I didn’t see any bands from Symbiodiniaceae in my gel 😦 I did see a band for coral tissue slurry, though, which means my PCR worked, but my samples didn’t have Symbiodiniaceae DNA. I’m going to change my filtering method to see whether I’m losing Symbiodiniaceae cells in my filtering process.

I didn’t see any 300 bp bands in my gel besides the coral sample, which is the fourth to last well.
Tuesday, July 29
A big storm is set to hit Mo’orea tomorrow, so we couldn’t go out on the boats today due to the high current. This means that I wasn’t able to get more rori for another coral feeding trial this week. Instead, I spent the day cleaning my tanks in preparation for new rori next week and prepping tubes.


I labeled lots of tubes to get them ready for full sampling in situ (in the water at the study site) on Friday!
I also helped my labmate with her sampling, which involved this really fancy light. We use this chemical/dye called PMA, which binds to DNA of broken/dead cells. We can use this to get an idea of the amount of live versus dead cells of certain microbes in our samples.

When we expose our samples combined with the dye to this fancy blue light, it binds to the DNA of dead cells.
Last and potentially most importantly…I went to the grocery store and picked up some essentials (a new dress and some cookies)! Have you ever tried these cookies?



I got a new dress and cookies from the grocery store.
Monday, July 28
Today I started prepping things for my big sampling days on Friday and Sunday. First, I walked down the hill from my bungalow to the lab. I had a friend join me today!

Chewy joined me on my walk down the hill today!
Once I was in the lab, I sterilized some tubes by putting them in a high-pressure, high-temperature machine called an autoclave. I put special tape on the tubes that gets black stripes to indicate the autoclave worked.

I sterilized/cleaned tubes in preparation of sampling soon.
After this, I counted Symbiodiniaceae cells in some of the samples from my coral feeding trial yesterday. I found some good-looking (live) cells, but my phone wouldn’t focus to take a photo in the microscope (if you know the struggle, you know the struggle).
I ended the day by extracting DNA from some of my samples from the feeding trial. I want to see if I detect Symbiodiniaceae DNA in my rori poop samples. I’ll test this out soon…so stay tuned!
I also went on a short run in the afternoon before it started raining. We’re expecting a big storm to hit starting tomorrow through Thursday, so I’m glad I could in a short run before the rain started!

It’s starting to look stormy outside!
We went out to eat tonight! It was great to have a real meal 🙂

I (finally) got a real meal for dinner.
While I was doing all this prep/lab work today, my labmates went out on the boat to do some rori surveys (i.e., see how many and what species of rori are around the island). They found a MASSIVE rori!!

My labmates found a giant sea cucumber today (plus adult human for size reference).
Sunday, July 27
After I walked down to the lab this morning, I was rewarded with a greeting from none other than miss Chewy.

Chewy greeted me this morning.
Then, I did another troubleshooting coral feeding trial. The main thing we learned from this trial is that (1) it takes FOREVER to sample these poops/sediments, so it’s gonna be all hands on deck for the real sampling! I also (finally) found a good filtering system after troubleshooting with many different types of mesh sizes. It’s important for me to filter my sediment samples because there’s a lot of non-Symbiodiniaceae junk that makes it difficult to count Symbiodiniaceae cells in the microscope. I’m so glad I found a system that works, no matter how slowly it filters!

I found a good method to filter my sediment/sand/poop samples.
Saturday, July 26
This morning, I went back to my site to test collection methods of rori poop. I found a new method that works great!


I snorkeled at my site and tested collection methods!
Then, I checked whether I found Symbiodiniaceae cells in my coral tissue and rori poop and yes (!) I found both live and dead Symbiodiniaceae cells in both 🙂 I still have some more troubleshooting to do, but I made great strides today! Stay tuned, because tomorrow I’m planning to retry my coral feeding trial!

I’m looking for the brown-ish cells in my rori poop samples!
After I processed some samples, we went to the Heiva in Tahiti, which is a cultural event in French Polynesia. Today was the last day of the event, and we saw chants and dances from winners of previous Heivas. It was very beautiful, and I’m so grateful to have had this experience!

We went to the Heiva in Tahiti (NOTE: this photo was taken after all performances).
As if this day couldn’t get any better, it ended with the most beautiful sunset:

The sunset was beautiful. Do you see Mo’orea? 😛
Friday, July 25
Today I found an amazing site with lots of sea cucumbers and live coral! I’m excited to sample there for my project 🙂 I went there in the afternoon to test sampling some rori poop. We got lots of rori poop samples, but we had trouble processing them. Tomorrow, I’m going to test new collection/filtering methods. But (!) I also collected three more rori – yay! I’m going to test out another coral feeding trial soon!

The view from the boat is great!

We found lots of rori pooping and collected some!
This trip, I also have a very important side quest to figure out how to market sea cucumbers to those who are more worm-averse. In lieu of tiny hats…what do you think of these angles?


Are these rori close-ups more charming?
Thursday, July 24
We had a very early start today! We left the station at 5:15 am to catch the first ferry from Mo’orea to Tahiti because we spent most of the day visiting a sea ranch on Tahiti!!


We enjoyed the sunrise on the ferry from Mo’orea to Tahiti.
The sea ranch is part of Tahiti Marine Biotech and Ifremer, so it’s a French research institution that farms endangered sea cucumbers for pharmaceutical development, scientific research, and eventual release/repopulation of endangered, historically overharvested sea cucumbers. This was a super cool experience because we got to talk to experiments in holothuriculture who are at the forefront of sea cucumber research, including sea cucumber gut microorganisms.

The sea ranch is part of Ifremer, a French research instution.

The rori are raised in these pools. The rori are in those green and black nets! Little fish outside the nets eat jellyfish.
After we saw the overall holothuriculture setup, we got to see rori BABIES! So cute! In the photo below, you can see different ages of 1-2 months, 4-6 months, and 1-1.5 years.

We got to see tiny rori babies!


We even got to hold the baby rori! In these photos, I’m holding a rori that’s 4-6 months old.
We also got to see the full-grown rori!


These rori get huge! This is the endangered Holothuria whitmaei.
We learned all about the lifecycle of H. whitmaei.
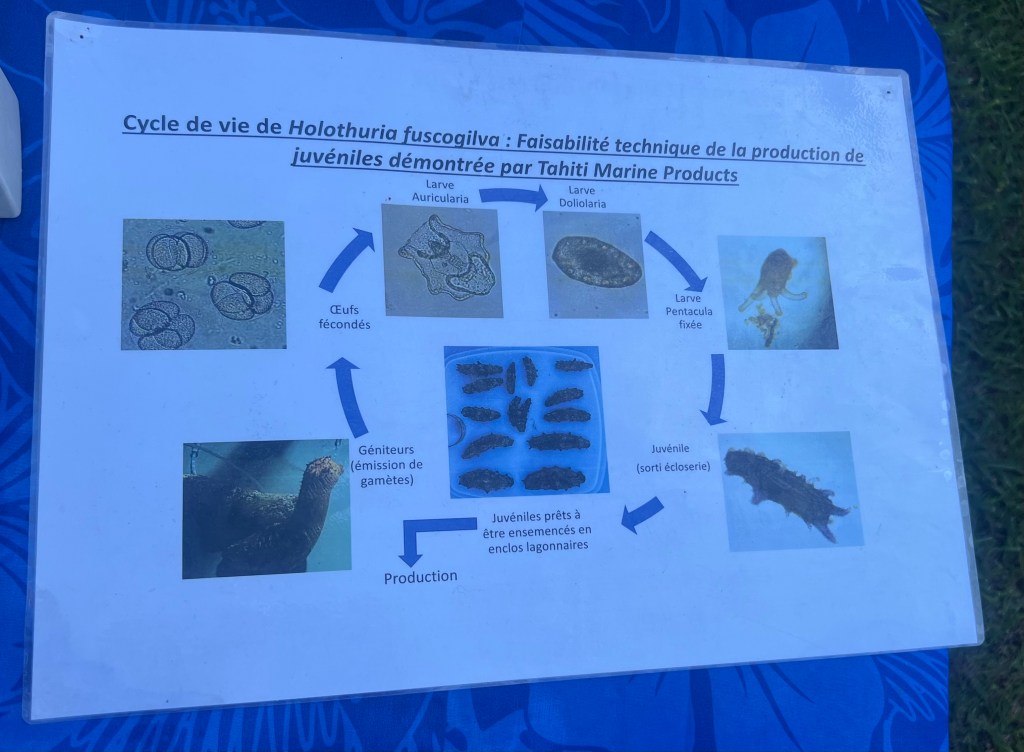
Rori grow from larvae to relatively giant adults.
We ended our visit with a presentation from the scientists at Tahiti Marine Biotech!

The folks at Tahiti Marine Biotech gave us a talk about their organization, scientific endeavors, and the cosmetic products and pharmaceuticals they’ve been able to make with sea cucumbers.
After the visit to the sea ranch, I ran my first coral feeding trial!! This was so fun and exciting, but it took until 9 pm, so it was a very long day. We started out by airbrushing the tissue off of a coral fragment. Then, we homogenized the slurry, fixed some of it for downstream analyses (cell counts and DNA extraction/sequencing), and fed some of it to a rori. I fed it to the rori by putting it into a syringe (without a needle), inserting the syringe into the rori’s mouth, and slowly pushing the coral slurry into the rori’s mouth. This slurry contained millions of Symbiodiniaceae cells, so I’m really excited to see if there are live Symbiodiniaceae cells in the rori poop after eating the slurry!

I started my first preliminary coral feeding trial by cutting off a branch tip of Acropora pulchra and feeding the tissue slurry to the rori.
After I fed the coral tissue slurry to the rori, I monitored it until it pooped. I sampled every poop for 2.5 hours after I fed it the tissue slurry! Stay tuned to see how the samples look tomorrow!
Wednesday, July 23
Today I ran another residence time trial with the smaller rori, H. atra. I put it in the tank with blue sand and measured how long it took to see blue sand in its poop. How long do you think it took this guy to poop out blue sand (hint: less than the larger rori…)? Since it took less time than the other rori, this was great news for my experimental design! We might not have to monitor the rori for as long as I originally thought after feeding it coral and/or cyanobacteria 🙂

Yummy yummy! The rori was enjoying its blue sand snack.
After I did these feeding trials, we had an outreach event with a local after-school program. A group of about 15 students ages 3-12 stopped by the station for a couple of hours. We gave them a tour of the station, showed them some fun creatures (including the pufferfish and the rori!), and had some activities where they learned about dive gear/signals, laying transects, and using a microscope. After the event, the kids thanked us by performing a song and dance! It was a great way to connect and give back to the community here in Mo’orea! Amazingly unplanned, the outreach day ended with a lemon shark siting right off the station!
Tuesday, July 22
Sea cucumbers have a lot of really cool defense mechanisms! One mechanism is evisceration, where they shoot out some of their internal organs to scare away things that are trying to eat or threaten them. Since I’m interested in microorganisms in their poop, I’m also interested in microorganisms in their guts. Sometimes, people dissect (and kill) them to access their gut contents. However, I would love to avoid killing a bunch of sea cucumbers! So, I’m testing a protocol to induce evisceration. Injecting KCl into the sea cucumber can stimulate its nervous system, causing it to eviscerate its guts. I started trying this method today, but I haven’t been successful. More to come on that…
…but for now, I also went out on the boat today to collect more rori and coral! First, I stopped to drop off the rori I collected the other day. I wanted to try to find a smaller species of sea cucumber called Holothuria atra. We found a site with tons of this species! After we returned the rori I previously collected, I collected two more small rori and a small branch of coral for a feeding trial.

I collected two smaller rori named Squirmy and Wormy. Wormy is shown above!
Monday, July 21
Since I’m interested in microorganisms in sea cucumber poop, I’m also interested in how long microorganisms spend in the sea cucumber gut before defecation (i.e., residence time). To measure residence time in sea cucumbers, I started running some preliminary experiments seeing how long it takes colored sand to appear in rori poop after consumption. Today, I ran my first trial of this experiment. First, I filled one of my tanks with a layer of blue sand in the top of one half of the tank.

I put blue sand in one half of a tank to see how long before the blue sand appears in the rori poop after consumption.
Then, I put one of my rori into the tank with blue sand. It almost immediately started eating it! You can even see specks of blue sand in the rori’s mouth in the photo below.


I put a rori into the tank with blue sand…
…and it started eating the blue sand!
After the rori ate the blue sand, I watched it until I saw specks of blue sand in its poop. How long do you think it took (hint: less than 24 hours…)?

After the rori ate the blue sand, it pooped it out! This tells me how long things are in the rori’s gut before being expelled in its poop.
After the first poop with some specks of blue sand, other poops had more blue sand. Eventually, I’ll do a larger experiment where I feed the rori coral tissue slurry and/or cyanobacteria and monitor their poop for signs of certain microorganisms. So, I’m using these residence time trials to inform my experimental design. For example, I think doing a time series sampling every poop after consumption of coral/cyanobacteria for a period of time will be helpful for my future experiment!
Sunday, July 20
We spent the morning doing some much-needed organizing in the lab. Then, I added sand to the tanks so we could get some rori in them.


We collected sand to put in the tanks on the water table.
We put a few inches of sand in the bottom of the tanks in the water table. After we added water, they were ready for rori!
We went out on the boat and collected some rori to put in the tanks! We found three rori total.


We found three rori for our tanks, two of which are shown above!
After that, I was showing off our rori to my partner on a video call when all of a sudden…one rori (to the right above) started pooping!!!! I ran to grab some tubes from the lab and began troubleshooting some collection methods. First, I tried using a whirlpak to collect the poop, but this didn’t work because it dissolved so immediately. Then, I tried doing a sort of punch biopsy of the poop with a 1.5 mL Eppendorf tube, and that worked! My goal was to collect poop without collecting water or sand in the tube. I put the 1.5 mL tube in the water with the cap closed so water can’t get in. Then, I open it upside down so the air stays in the tube and water stays out. I close the tube over the poop, and voila! We can sample the poop without collecting water 🙂

One of the sea cucumbers started pooping!! It’s poop is the light colored sandy material to the left of the tank shown above.
I preserved the poop in 10% formalin in 0.02 µm filtered seawater. This allowed me to look at live/dead Symbiodiniaceae cells under the microscope after I stained it with Trypan Blue.
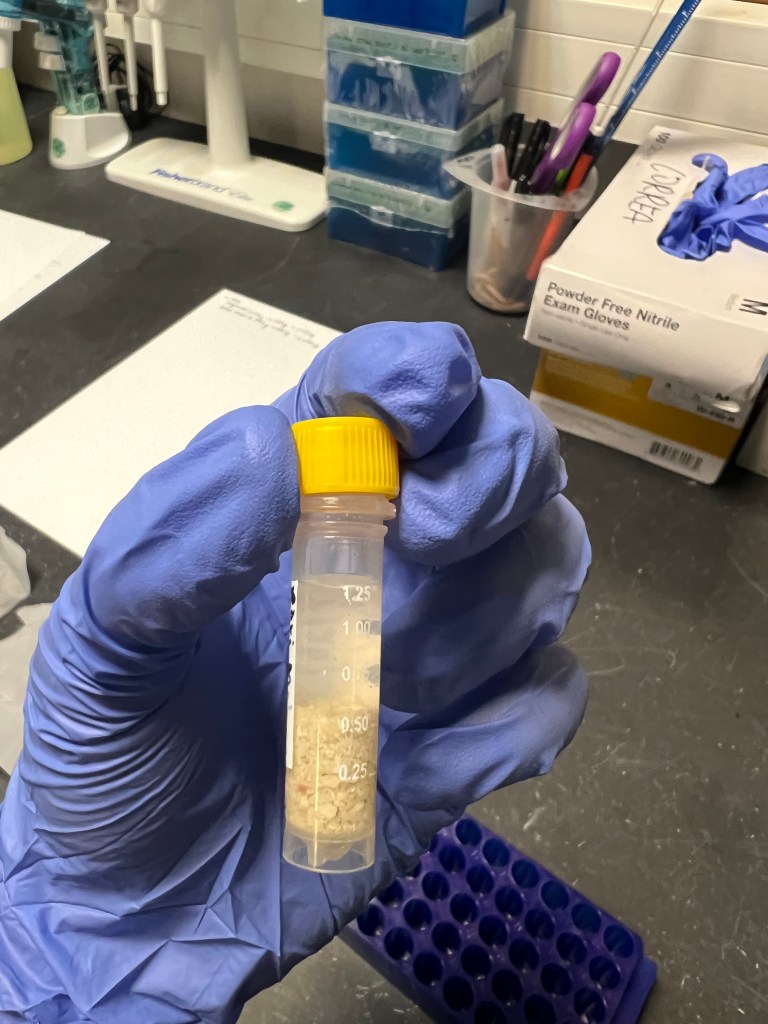
After I collected the rori poop, I transferred it to a 2 mL cryogenic vial with 750 µL of 10% formalin in filtered seawater to preserve any potential Symbiodiniaceae cells.
After I preserved the sample, I stained it with Trypan Blue so I could see live and dead cells under the microscope. Live Symbiodiniaceae cells are about 6-12 µm diameter, round, and have an orange-brown color. Dead Symbiodiniaceae cells have a blue circumference. I think I found a Symbiodiniaceae cell in the rori poop!! That’s super exciting, and I can’t wait to keep exploring this. I’m planning on lots of fun tank experiments tomorrow, so be sure to stay tuned!

I think I potentially found a Symbiodiniaceae cell in the rori poop! It was hard to take an image through the microscope, but the potential cell is the circular, orange-ish structure directly out from the arrow in the bottom right.
Saturday, July 19
For part of my project, I’m interested in having tank experiments with rori. Today, I set up some of the tanks for this portion of my project. Our lab recently got a shipping container at the station, which is really exciting because we have a lot more storage space now! So, I first got the glass tanks from the container.

Our lab recently got a shipping container for storage. We share the container with another lab that works at the station.


I cleaned four glass tanks to use in experiments with rori.
I set up the water table for the rori tank experiments. This is the before!
We also set up one of our filtration systems (tangential flow filtration or TFF). The TFF system basically functions by passing a sample through a filter cartridge that’s basically rolled up chicken wire. Without pressure in the system, the sample runs straight through the cartridge and isn’t filtered because it doesn’t permeate the filter. Once we apply back pressure, though, the sample passes through the filter, so we can make filtered seawater! We can also use this system to concentrate samples.

We set up tangential flow filtration (TFF).
We also went to a shore access site about 2 miles from the station and found some rori! We watched the rori until one pooped. Then, we sampled the rori in a 50 mL conical tube. However, we learned that the poop dissolves pretty immediately. I wanted to use that sample to see whether we see little algae that live inside coral (in the Family Symbiodiniaceae) in the rori poop. However, I couldn’t say whether the Symbiodiniaceae would come from the water or the sediment (or the rori poop), so I decided not to use this sample. Tomorrow, we’re going to troubleshoot other sampling methods! Stay tuned 🙂

We found sea cucumbers and watched them until they pooped!
Friday, July 18
Today I began my third field season in Mo’orea, French Polynesia at the UC Berkeley Gump Research Station with the Correa Lab. I’m with a large group with projects ranging from sea cucumbers (rori in Tahitian), to macroalgae (seaweed, or remu in Tahitian), to submarine groundwater discharge. We transported all 10 (!) of our bags from Tahiti to Mo’orea after enjoying some delicious chocolate croissants at the ferry station.



We brought 10 bags with us to Mo’orea, including 6 giant gear bags!
We ate chocolate croissants before taking the ferry from Tahiti to Mo’orea.
The Aremiti ferry has a beautiful view of Mo’orea!
Once we made it to Mo’orea, we got settled into our bungalow. We have to climb up a super steep hill to get there, but it comes with amazing views!


We have to climb up a very steep hill to get to the bungalow we’re staying in…
…but the climb is well worth it with this incredible view!
We spent most of the day unpacking all of our bags and getting our lab bench set up and, of course, enjoying the beauty of Mo’orea.

The dock has a beautiful view of the lagoon! Can you find the sleeping man? Hint: look at the mountain ridge…
The highlight of my day by far was…SQUISHY! I got to feed a pufferfish a coral frag from my hand! 🙂
