August 12-19, 2023
For the last week of our trip, we finished up all of our projects and cleaned up our experiments. This meant we spent a lot of time in the water, so I couldn’t take a lot of photos.
However, I’ve taken a lot of non-science photos throughout my time in Mo’orea. I’m so grateful for the opportunity to travel to such a beautiful part of the world, learn about new cultures, engage with other communities, do such cool research, and collaborate with other amazing scientists and community members! I’m also very grateful to be able to share my experiences with others through this blog! Please feel free to use the Contact page of my website if you want to reach out with any questions or if you want to connect. Thanks so much for following along with me this field season, and I’ll leave you all with some beautiful photos of the island of Mo’orea 🙂







August 11, 2023
For the remainder of the trip (including today), we’ll be spearing more fish and collecting environmental samples for the comprehensive assessment of Symbiodiniaceae. This means that we’ll get plenty of time to explore Mo’orea’s amazing coral reefs! 🙂
We also have plenty of photogrammetry photos to take and cleaning up to do, so stay tuned to see how the end of my trip will pan out!
August 10, 2023
We collected more giant clams today! This is really exciting so we can complete more of the giant clam project in a few days.

While the clams were acclimating, we collected more samples for my labmate’s comprehensive assessment of Symbiodiniaceae across Mo’orea’s north shore. I also took a lot more photogrammetry photos!
August 9, 2023
Today our first clam finished incubating! We moved it into filtered seawater to start collecting fecal pellets. While the clam was in the filtered seawater for five hours, I worked on completing paperwork to allow us to import samples to the US after our trip. We leave in a little over a week, so it’s really important to start working on the necessary paperwork now!
After five hours, we saw fecal pellets in the container the clam was in! They look like really small, brown specks in the container.

After we collected the fecal pellets, we filtered them and fixed them to 3% formalin, which will allow us to analyze the prevalence of sexual reproduction at a later date.

August 8, 2023
This is one of my labmate’s last days here in Mo’orea! We’re so glad we were able to accomplish so much with her throughout the trip, though! We started out the day by going on a sunrise hike with a member from the Vega-Thurber lab.

Then, we started taking photos for photogrammetry. This is a very important process because it allows us to get accurate measures of Symbiodiniaceae density in a coral fragment. First, we take about 140 photos of a coral fragment at two different angles. Then, we put all of the photos into a program that creates a model of the coral fragment. We use the model to measure the surface area of the fragment, which allows us to calculate the density of live Symbiodiniaceae within a coral fragment.

After we took photos of each fragment, we put the fragments in bleach to store them. It’s important to keep all of the fragments even after we’ve taken photos so we can retake photos in the future in case the program is unable to create a model.

August 7, 2023
Today, we finished sampling for the menthol bleaching experiment. So, we analyzed each fragment using the iPAM fluorometer, Amscope, and color watch card. Then, we airbrushed off the tissue from the coral skeleton so we can do more analyses after the trip. We’ll be looking at the density of Symbiodiniaceae between treatments, so we expect to see the greatest density of live symbionts in the fragments that received fresh fish feces treatments if the fish feces transmit Symbiodiniaceae to coral.
We spent the rest of the day taking down the experiment and cleaning up the room we were using.
August 4-5, 2023
For the past couple of days, I’ve been collecting samples from other sites for the project we started on August 3. This means I’ve been collecting more environmental samples and spearing fish!
However, today (August 5) we started our fourth and final project! I’m mentoring an undergraduate student on a project investigating meiosis in Symbiodiniaceae found in giant clam fecal pellets. After 40 minutes with the hammer and chisel, we finally collected our first clam today!! We’re all super excited to get this project started and I can’t wait to see what results we see. The clam is currently acclimating to the tank, but we’ll start collecting fecal pellets very soon 🙂

August 3, 2023
Today we started collecting samples for my labmate’s experiment, which is a comprehensive assessment of Symbiodiniaceae across Mo’orea’s north shore. To do this, we went to one site to do a transect to assess coral cover. Then, we collected environmental samples (i.e., water, sediment, macroalgae, and coral). We tried to do fish follows and catch a few fish to collect their feces, but we couldn’t find the species we were interested in. This means we’ll have to return to the site in a few days to do the fish follows.
After we collected the samples and brought them back to the lab, we started processing all of the environmental samples. The first step was filtering the sediment samples through a 120 µm followed by a 25 µm mesh.

Then, we filtered the water samples through a very small mesh using the vacuum pump.


We also scrubbed epiphytes off of the macroalgae samples.


Last, we used an airbrush to remove the coral tissue from the skeleton.

Overall, it was a successful first day of sampling, and we have a lot more to do to finish collecting samples from all eight sites. Stay tuned to see how sampling goes tomorrow 🙂
August 2, 2023
I spent today helping my labmate apply the final treatment for her menthol bleaching experiment. First, we went out to catch a fish for the feces treatments while our other labmates went out to collect more crabs. This time, we collected a different butterflyfish species, Chaetodon lunulatus.

After we dissected the fish and extracted its feces, we spent the rest of the day applying the treatments to the coral fragments for the menthol bleaching experiment. Before we applied the treatments, we checked the status of each fragment by comparing it to the coral watch card, which is a standard card used to evaluate coral bleaching.
Tomorrow we’re going to start sampling for the other two projects we’re working on this trip. Fingers crossed we can find some giant clams and spear enough fish for my labmate’s project!
July 30, 2023 – August 1, 2023
The last couple of days have been really hectic! I helped my labmate get another fish for the second treatment for her menthol bleaching experiment on July 30.
On July 31, I checked on the corals for my heat stress experiment in the morning. Unfortunately, several colonies were showing signs of bleaching and mortality. This meant that I had to quickly prepare for immediate sampling. We started sampling at noon on July 31 and sampled every two hours for 24 hours, with 10 am on August 1 as the last timepoint. Unfortunately, I’ll have to redo the experiment due to issues relating to coral mortality and improperly fixing certain samples for my analyses. Though this is disappointing, this is common in the field and I’ve learned a lot for future iterations of the project! I didn’t have much time to take photos, but here are a few of me sampling during the 24-hour sampling day 🙂


July 29, 2023
I spent today mostly preparing to process samples for my experiment. On August 6, we’ll sample every two hours for 24 hours. To streamline the process, I’ve been working on labeling all the equipment we’ll need. Today, I labeled all the whirlpaks and sorted them by timepoint. We’ll use these when we airbrush off all of the coral tissue. I also started labeling 50 mL falcon tubes which I’ll use to fix the tissue slurry in. However, I didn’t get too far with this since I have to label 600 tubes.


I also checked on the corals for my experiment. They seem to be doing well, which is great! The water is slowly heating up to the ideal temperature for my heat stress treatment. I did have to lower the water level, though, to allow the water to heat up enough.

I ended the day by helping one of my labmates troubleshoot some filtering processes for her project. She wants to do a comprehensive assessment of Symbiodiniaceae across the north shore of the island across different nutrient gradients, so part of her project involves collecting environmental samples (i.e., water, sand, and macroalgae). I was helping her troubleshoot a gravity filtering system for the sediment samples. First, I filtered sediment through a 120 µm mesh before filtering through a 25 µm mesh. Then, she used a vacuum pump to filter through an even smaller mesh.

July 28, 2023
The corals for my heat stress experiment are acclimating today, so I helped my labmate with her menthol bleaching experiment. She’s interested in whether corallivorous (i.e., coral-eating) fish feces transmit Symbiodiniaceae to corals, so she is applying fish feces to the bleached coral fragments. Today was the first treatment day, so we went out and caught three fish so we could dissect them for their feces (DRM permit number 2022030; APA permit number 2022012).

After we caught and dissected the fish, we added the appropriate treatment to each fragment. Some fragments received raw seawater, others received an ectosymbiont (a cute little crab), others received fresh feces, and some even received sterile feces. The final setup looked something like this:

July 27, 2023
Today was the official start of my experiment!! I’m super excited to get things started and can’t wait to see the results down the line. As a reminder, I’ll be doing a heat stress experiment to see whether elevated temperatures induce shifts from asexual to sexual reproduction in coral-associated Symbiodiniaceae. I started out by troubleshooting the tank heaters. I tested two smaller heaters and one large heater to decide how I want my final experimental setup to be once I’m applying heat treatments.


Once I got an idea of how the heaters affect the temperature, I created some labels for our sampling. Sampling will take place every two hours for 24 hours, so each label has the project name followed by the colony ID, timepoint, and treatment. Once we sample, I’ll add the date to each label.
Then, in the afternoon we collected six coral colonies for my project!! They’re currently acclimating in their flow-through system. Tomorrow, I’ll move them to their treatment tanks so we can prepare to start applying the heat treatment.


July 26, 2023
Today we started the day by scouting out some sites for my labmate’s project. She needs eight sites around Mo’orea’s north shore with specific fish species. We located two potential sites with shore access!


After that, we moved the coral fragments for the menthol bleaching experiment into tripours, which is where they will receive their respective treatments. We followed the same processing protocol as we did on July 24 (i.e., iPAM, Amscope, airbrush, etc.). However, I had to spend a lot of time figuring out the tripour setup with all of the bubblers! I connected each bubbler to a glass pasteur pipette inside each tripour.

I spent the rest of the day preparing for my heat stress experiment, which we will start tomorrow! First, I siphoned out the glass aquaria to clean them.


Last, I ended the day by putting in a tank heater to test it out before applying the heat treatment. My goal is to elevate the heat by 3°C, so I’m going to monitor the temperature in the tank with the tank heater overnight to ensure I know the proper settings. To do this, I put in a temperature/light monitor called a hobo tag, which will allow me to collect this data every 15 minutes! I can’t wait to collect corals for this experiment tomorrow! 🙂

July 25, 2023
This morning, we applied the second menthol treatment to the coral fragments. We also had to move to a bungalow at the research station, so I had to say goodbye to my favorite island cat, S’mores! However, I’m excited to wake up to the beautiful bungalow view every morning for the remainder of the trip.


Once we finished moving to the bungalow, I spent the rest of the morning troubleshooting our bubbler system for the menthol bleaching experiment. These are important to supply airflow to the corals since they will be in tripour containers without flow-through to avoid cross-contamination between treatments. I figured out a system to generate air for three tripours with one bubbler, although the system isn’t perfect.


Before we left for the day, we moved the coral fragments out of the menthol solution to let them rest before the next treatment tomorrow morning. We’re monitoring how bleached they are after we apply each treatment, and you can see some of the fragments below. They are looking a lot whiter, which is indicative of bleaching! Tomorrow, we plan to apply the next menthol treatment and scout out some sites around the island for my labmate’s project.
July 24, 2023
Today was the official start of the menthol bleaching experiment! I woke up early to help apply the menthol treatment to the coral fragments so they will bleach. The treatment has to sit for eight hours before the fragments are moved out of the menthol treatment. We’ll apply menthol treatments for the next few days, too.

After the menthol treatment, we sampled one fragment per colony for our initial timepoint. This will allow us to have base measurements at the start of the experiment before the application of the treatments so we can see how the treatments affect the corals. This consisted of multiple steps. First, we used an iPAM fluorometer to measure the photosynthetic capacity of the Symbiodiniaceae in the coral fragments. Then, we used the Amscope to take a photo of each coral fragment before taking a clip of each fragment for DNA extractions to analyze the microbial community in the coral. Last, we airbrushed the tissue off of each fragment so we could take samples to measure the macromolecules and Symbiodiniaceae density in the corals. You can see some of this setup below!





July 23, 2023
Today, we prepared a tank system for the first portion of the upcoming experiment. We set up bins with bubblers in them so we could apply menthol treatments to the coral fragments. This causes them to bleach, a process where they expel the Symbiodiniaceae living inside of them.

After that, I made labels for each rack full of coral fragments. This will help us keep track of each fragment so we know which one we are sampling from throughout the experiment.

Then, I helped feed the crabs that we’ll be using in the experiment! To do this, we gave them spare small coral fragments.

We ended up finishing all of that around lunchtime, so we spent the rest of the day snorkeling and working on our coral and fish IDs. Tomorrow, we’ll start applying the menthol treatments for the official start of the menthol bleaching experiment!! Check out the photos below for some of the local fauna here in Mo’orea 🙂









July 22, 2023
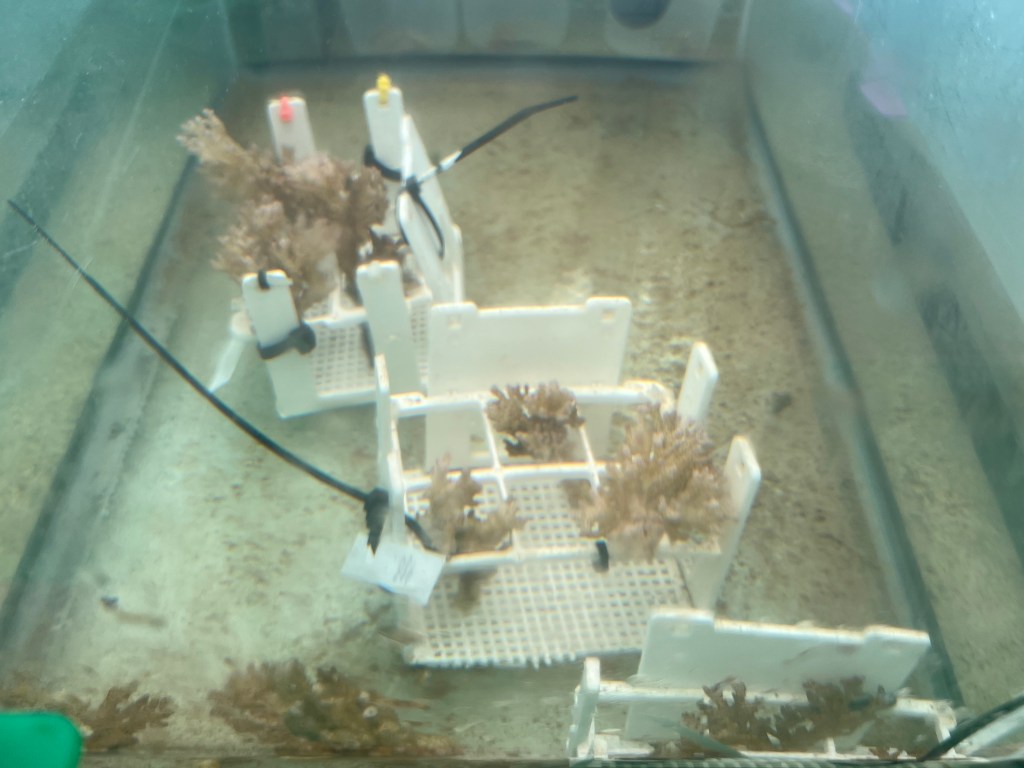
This morning, I started the day by helping set up some of the tanks for upcoming experiments. We’ll use six tanks with flow-through to maintain coral colonies. The photo below isn’t the final product, but you can get an idea of what the setup looks like.

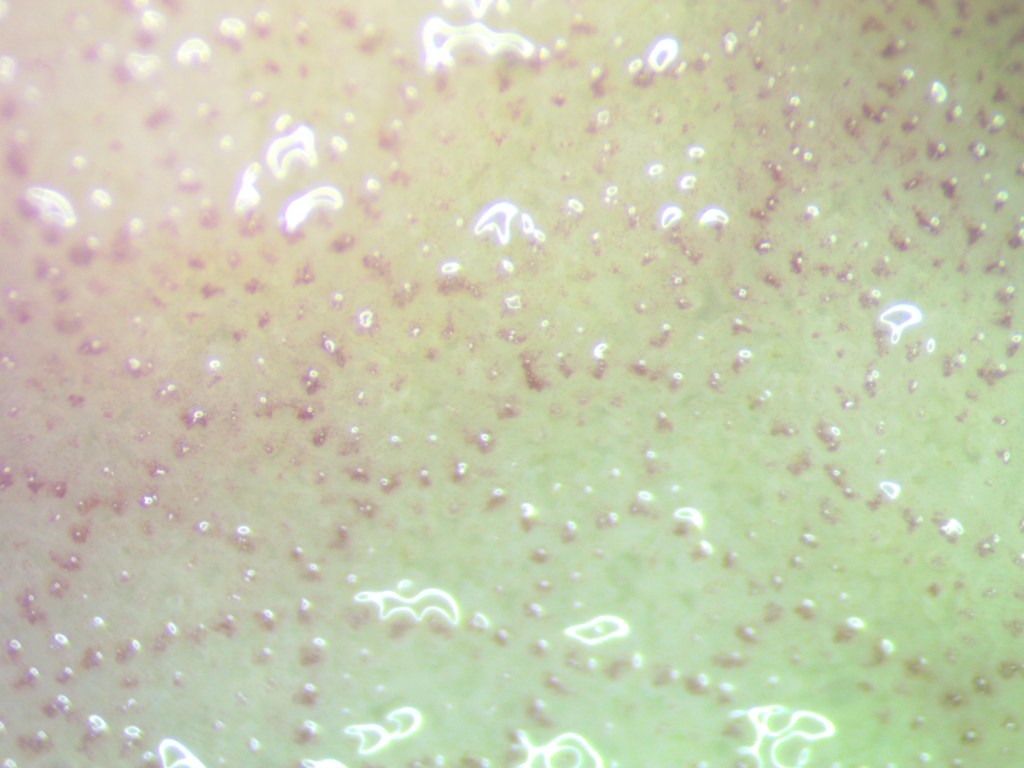
Then, I set up the microscope that we use to take close-up photos of coral fragments. This helps us monitor the coral fragments throughout the experiment so we can see whether or not they have Symbiodiniaceae living inside them! You can see an example of what a close-up photo might look like below.

Lastly, I ended the day by helping my labmates collect six coral colonies for an experiment. Then, we split each colony into at least nine smaller fragments that will be divided among different treatments. I can’t wait to get the experiment started, but until then we’ll let the corals acclimate to their new environment. 🙂

July 21, 2023
I got started this morning by greeting Skippy, one of the station dogs!

Then, we got to work finding some old samples to continue troubleshooting our method to preserve coral fragments.

After that, I started prepping our experimental setup. We’re going to put 36 coral fragments in individual containers.

In the afternoon, we took the boat out and collected two coral colonies for the experiment! Tomorrow, we’ll get four more colonies, so stay tuned for some cool coral pictures.
July 20, 2023
Hello again! Today we started setting getting ready for our first experiment, which will test whether coral-eating fish feces supply symbionts to corals. I spent all morning troubleshooting our tangential flow filtration system to generate filtered seawater that is free of microorganisms like bacteria and Symbiodiniaceae. To do this, raw seawater is pumped through a 0.2 micron filter as shown below.

Then, I had a quick lunch break where I found a furry friend borrowing my shoe. I love all of the animals here at the research station!



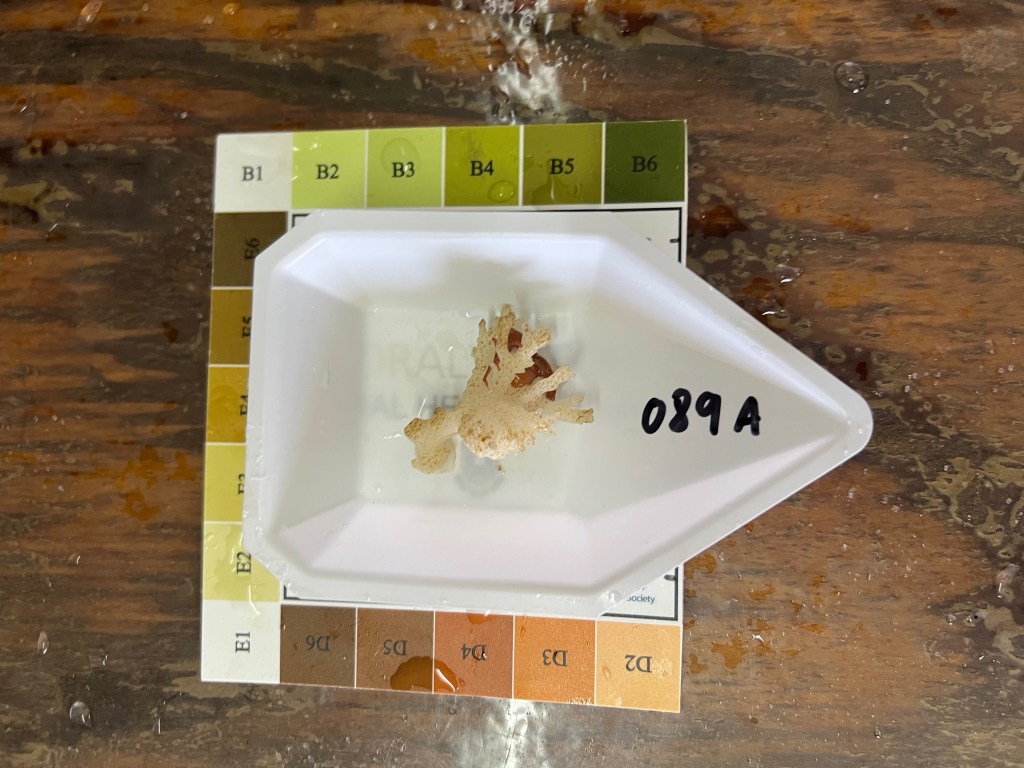
After lunch, I cleaned out some tanks for the upcoming experiment. Fingers crossed we can collect some corals tomorrow! I also started troubleshooting a protocol to preserve coral fragments using one of our samples from a previous field mission, shown below.

I also started troubleshooting other protocols for the upcoming experiment. I started setting up the bandsaw so we can cut coral colonies into smaller fragments. That’s all for today! I’ll leave you with my view as I write this blog! 🙂

July 19, 2023
Hello everyone! I’ll be documenting my fieldwork in Mo’orea, French Polynesia at the Gump Research Station on this page. This summer, I’m spending a month in Mo’orea with three of my labmates. We’ll be working on four different projects, one of which is for a chapter of my PhD dissertation! I’m interested in microalgae that lives inside reef-building corals in the family Symbiodiniaceae, so I’m performing a heat experiment to test whether environmental stress triggers shifts from asexual to sexual reproduction in the coral-associated Symbiodiniaceae.
Yesterday, I flew from Houston, Texas to Papeete, Tahiti. I stayed in a hotel in Tahiti overnight before taking the ferry from Tahiti to Mo’orea this morning. Unfortunately, it was pouring rain today, even though it’s the dry season in French Polynesia.



After we arrived at the research station, we spent a lot of time unpacking our six bags of research equipment! We’re getting organized to start working on our experiments tomorrow.
